Daniel Colón-Ramos, PhD
Dorys McConnell Duberg Professor
of Neuroscience and Cell Biology
Department of Neuroscience
Yale Univeristy
(September 8, 2020)
Molecular mechanisms synaptic assembly and function: Lesson from C. elegans
Learning and behavior are closely linked and are an essential component of survival. We learn very quickly to avoid a hot stovetop, after only one painful experience. In the brain, learned behaviors depend on changes in the connections between neurons, the synapse. Dr. Colón-Ramos discussed the interplay of two plasticity (change) mechanisms in the worm C. elegans. His work has demonstrated that both adaptation and plasticity occur in a single cell to guide behavior.
 My laboratory is interested on the cell biology of the synapse. When, where and how synapses form underpins behaviors. We have focused our studies in understanding the assembly, maintenance and plasticity of specific synapses within neurons that form part of a tractable behavioral circuit in C. elegans: the thermotaxis circuit. C. elegans does not have an innate preferred temperature and can instead be trained to prefer specific temperatures given previous favorable experiences.
My laboratory is interested on the cell biology of the synapse. When, where and how synapses form underpins behaviors. We have focused our studies in understanding the assembly, maintenance and plasticity of specific synapses within neurons that form part of a tractable behavioral circuit in C. elegans: the thermotaxis circuit. C. elegans does not have an innate preferred temperature and can instead be trained to prefer specific temperatures given previous favorable experiences.
We used this system to examine how different plasticity mechanisms act together in vivo and at a cellular level to transform sensory information into behavior. We recently demonstrated that in the nematode C. elegans two plasticity mechanisms—sensory adaptation and presynaptic plasticity—act within a single cell to encode thermosensory information and actuate a temperature-preference memory. Sensory adaptation enables the primary thermosensory neuron, AFD, to adjust the temperature range of its sensitivity to the local environment, thereby optimizing its ability to detect temperature fluctuations associated with migration. Presynaptic plasticity transforms this thermosensory information into a behavioral preference by gating synaptic communication between sensory neuron AFD and its postsynaptic partner, AIY. The gating of synaptic communication is regulated at AFD presynaptic sites by the conserved kinase nPKC epsilon. Bypassing or altering AFD presynaptic plasticity predictably changes the learned behavioral preferences without affecting sensory responses. Our findings indicate that two distinct neuroplasticity mechanisms function together within a single sensory neuron to encode multiple components of information required to enact thermotactic behavior. The integration of these plasticity mechanisms results in a single-cell logic system that can both represent sensory stimuli and guide memory-based behavioral preference.
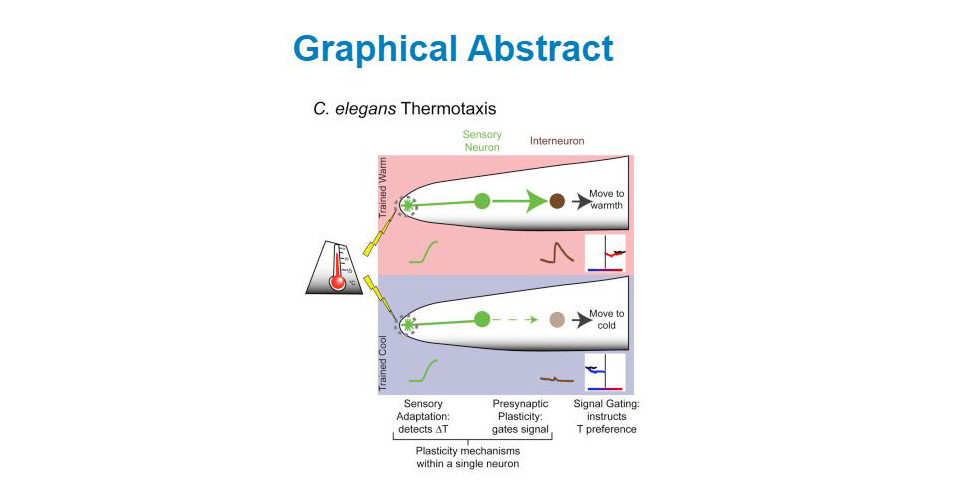
Visual abstract from Daniel Colón-Ramos' talk