Leslie Griffith

Nancy Lurie Marks Professor of Neuroscience
Director, Volen National Center for Complex Systems
Research Description
Biochemistry of Behavior
Survival requires that animals interact in appropriate and adaptive ways with their environment. My lab is interested in how the nervous system integrates information and generates behavioral outputs. We study behavior at the biochemical, cellular and organismal levels using Drosophila melanogaster as a model organism.
Flies, like humans, have basic behaviors (such as locomotion and reproductive behaviors) that can be altered by the animal’s internal state or by exposure to external cues. We use video and automated monitoring systems to assess locomotion, circadian rhythms, sleep and courtship. The ability to manipulate the activity and biochemistry of particular neurons with exquisite spatial and temporal control makes Drosophila a powerful system for this type of integrative approach.
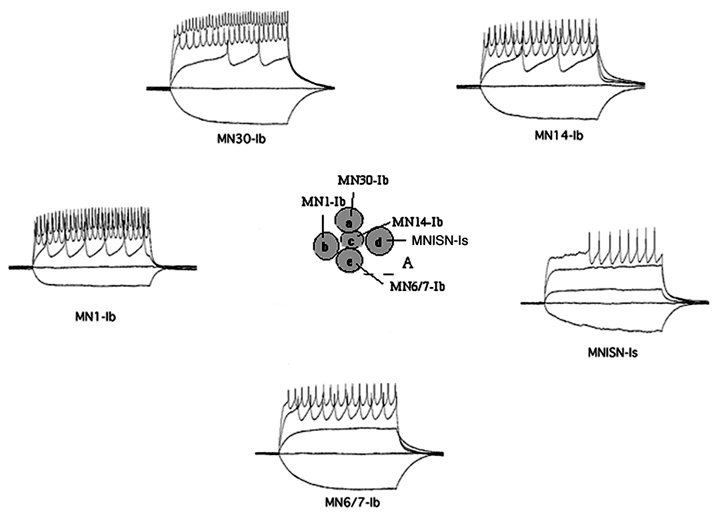
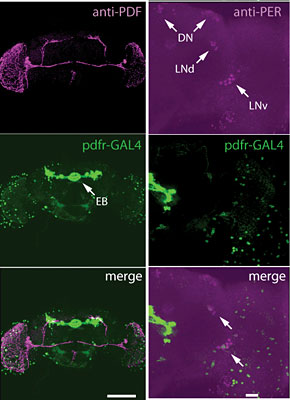 At the cellular level we use both imaging and electrophysiology to understand how the activity of neurons is changed by experience and how these changes are translated into behaviors. Imaging of calcium responses in the adult brain in the sleep circuitry has allowed us to understand the effects of neuromodulators and sleep deprivation on neuronal activity. Direct recording from central neurons in larvae and adults allows us to investigate how cellular activity is regulated during ongoing behavior or as a result of learning. At the biochemical level, we are interested in how calcium, a ubiquitous and critical second messenger, transduces information and regulates plasticity. One of the main mechanisms by which calcium acts within the cell is through the activation of calcium-dependent protein kinases such as calcium/calmodulin-dependent protein kinase II (CaMKII). CaMKII homologs have been found in all multicellular organisms that have been examined and have also been found in yeast. In Drosophila, we have shown that CaMKII is required for both behavioral plasticity in courtship learning and for normal synaptic function at the neuromuscular junction. The long-term goal of this research is to understand the signal transduction pathways underlying changes in synaptic structure and function. By starting with a protein kinase, we have been able to identify both upstream (regulators such as CASK, a MAGUK scaffolding protein) and downstream (substrates such as Eag) elements of the pathway.
At the cellular level we use both imaging and electrophysiology to understand how the activity of neurons is changed by experience and how these changes are translated into behaviors. Imaging of calcium responses in the adult brain in the sleep circuitry has allowed us to understand the effects of neuromodulators and sleep deprivation on neuronal activity. Direct recording from central neurons in larvae and adults allows us to investigate how cellular activity is regulated during ongoing behavior or as a result of learning. At the biochemical level, we are interested in how calcium, a ubiquitous and critical second messenger, transduces information and regulates plasticity. One of the main mechanisms by which calcium acts within the cell is through the activation of calcium-dependent protein kinases such as calcium/calmodulin-dependent protein kinase II (CaMKII). CaMKII homologs have been found in all multicellular organisms that have been examined and have also been found in yeast. In Drosophila, we have shown that CaMKII is required for both behavioral plasticity in courtship learning and for normal synaptic function at the neuromuscular junction. The long-term goal of this research is to understand the signal transduction pathways underlying changes in synaptic structure and function. By starting with a protein kinase, we have been able to identify both upstream (regulators such as CASK, a MAGUK scaffolding protein) and downstream (substrates such as Eag) elements of the pathway.
Selected Publications
- Rest Is Required to Learn an Appetitively-Reinforced Operant Task in Drosophila. Wiggin TD, Hsiao Y, Liu JB, Huber R, Griffith LC. Front Behav Neurosci. 2021 Jun 18;15:681593. doi: 10.3389/fnbeh.2021.681593. eCollection 2021.
- Availability of food determines the need for sleep in memory consolidation. Chouhan NS, Griffith LC, Haynes P, Sehgal A. Nature. 2021 Jan;589(7843):582-585. doi: 10.1038/s41586-020-2997-y.
- The Role of Dopamine in Associative Learning in Drosophila: An Updated Unified Model. Adel M, Griffith LC. Neurosci Bull. 2021 Jun;37(6):831-852. doi: 10.1007/s12264-021-00665-0. Epub 2021 Mar 29.
- MicroRNAs Regulate Multiple Aspects of Locomotor Behavior in Drosophila. Donelson NC, Dixit R, Pichardo-Casas I, Chiu EY, Ohman RT, Slawson JB, Klein M, Fulga TA, Van Vactor D, Griffith LC. G3 (Bethesda). 2020 Jan 7;10(1):43-55. doi: 10.1534/g3.119.400793.
- Covert sleep-related biological processes are revealed by probabilistic analysis in Drosophila. Wiggin TD, Goodwin PR, Donelson NC, Liu C, Trinh K, Sanyal S, Griffith LC. Proc Natl Acad Sci U S A. 2020 May 5;117(18):10024-10034.
- The conserved microRNA miR-34 regulates synaptogenesis via coordination of distinct mechanisms in presynaptic and postsynaptic cells. McNeill EM, Warinner C, Alkins S, Taylor A, Heggeness H, DeLuca TF, Fulga TA, Wall DP, Griffith LC, Van Vactor D. Nat Commun. 2020 Feb 27;11(1):1092. doi: 10.1038/s41467-020-14761-8.
- Regulation of Olfactory Associative Memory by the Circadian Clock Output Signal Pigment-Dispersing Factor (PDF). Flyer-Adams JG, Rivera-Rodriguez EJ, Yu J, Mardovin JD, Reed ML, Griffith LC. J Neurosci. 2020 Nov 18;40(47):9066-9077. doi: 10.1523/JNEUROSCI.0782-20.2020.
- A Serotonin-Modulated Circuit Controls Sleep Architecture to Regulate Cognitive Function Independent of Total Sleep in Drosophila. Liu C, Meng Z, Wiggin TD, Yu J, Reed ML, Guo F, Zhang Y, Rosbash M, Griffith LC. Curr Biol. 2019 Nov 4;29(21):3635-3646.e5. doi: 10.1016/j.cub.2019.08.079.
- MicroRNAs Regulate Sleep and Sleep Homeostasis in Drosophila. Goodwin PR, Meng A, Moore J, Hobin M, Fulga TA, Van Vactor D, Griffith LC. Cell Rep. 2018 Jun 26;23(13):3776-3786. doi: 10.1016/j.celrep.2018.05.078.
- Regulation of Eag by Ca2+/calmodulin controls presynaptic excitability in Drosophila. Bronk P, Kuklin EA, Gorur-Shandilya S, Liu C, Wiggin TD, Reed ML, Marder E, Griffith LC. J Neurophysiol. 2018 May 1;119(5):1665-1680. doi: 10.1152/jn.00820.2017.
- Nonreciprocal homeostatic compensation in Drosophila potassium channel mutants. Kim EZ, Vienne J, Rosbash M, Griffith LC. J Neurophysiol. 2017 Jun 1;117(6):2125-2136. doi: 10.1152/jn.00002.2017.
- The Long 3'UTR mRNA of CaMKII Is Essential for Translation-Dependent Plasticity of Spontaneous Release in Drosophila melanogaster. Kuklin EA, Alkins S, Bakthavachalu B, Genco MC, Sudhakaran I, Raghavan KV, Ramaswami M, Griffith LC. J Neurosci. 2017 Nov 1;37(44):10554-10566. doi: 10.1523/JNEUROSCI.1313-17.2017.
- Kim EZ, Vienne J, Rosbash M and Griffith LC (2017). "Non-reciprocal homeostatic compensation in Drosophila potassium channel mutants." J Neurophysiol: jn 00002 02017.
- Guo F, Yu J, Jung HJ, Abruzzi KC, Luo W, Griffith LC, Rosbash M (2016). "Circadian neuron feedback controls the Drosophila sleep--activity profile." Nature 536(7616): 292-297.
- Parisky KM, Agosto Rivera JL, Donelson NC, Kotecha S, and Griffith LC (2016). "Reorganization of Sleep by Temperature in Drosophila Requires Light, the Homeostat, and the Circadian Clock. "Curr Biol 26(7): 882-892.
- Haynes PR, Christmann BL, Griffith LC (2015). "A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster." elife 2015;4:e03868.
- Langenhan T, Barr MM, Bruchas MR, Ewer J, Griffith LC, Maiellaro I, Taghert PH, White BH, Monk KR (2015). "Model Organisms in G Protein-Coupled Receptor Research." Mol Pharmacol 88(3): 596-603.
- Liu C, Haynes PR, Donelson NC, Aharon S, Griffith LC (2015). "Sleep in Populations of Drosophila Melanogaster." eNeuro 13 August 2015, 2 (4) ENEURO.0071-15.2015).
- Chi MW, Griffith LC, Vecsey CG (2014). "Larval Population Density Alters Adult Sleep in Wild-Type Drosophila melanogaster but Not in Amnesiac Mutant Flies." Brain Sci 4(3): 453-470.
- Griffith LC (2014). "A big picture of a small brain." elife. 2014;3:e05580.
- Griffith LC (2014). "Neuroscience: What females really want." Nature 512(7513): 138-139.
- Slawson JB, Kuklin EA, Mukherjee K, Pirez N, Donelson NC, Griffith LC (2014). "Regulation of dopamine release by CASK-beta modulates locomotor initiation in Drosophila melanogaster." Front Behav Neurosci 8: 394.
- Vecsey CG, Pirez N, Griffith LC (2014). "The Drosophila neuropeptides PDF and sNPF have opposing electrophysiological and molecular effects on central neurons." J Neurophysiol 111(5): 1033-1045.
- Griffith LC (2014). "Up all night on a redeye flight." eLife 2014;3:e02087.
- Mukherjee K, Slawson JB, Christmann BL, Griffith LC (2014). "Neuron-specific protein interactions of Drosophila CASK-beta are revealed by mass spectrometry." Front Mol Neurosci 7: 58.
- Griffith LC (2013). "Neuromodulatory control of sleep in Drosophila melanogaster: integration of competing and complementary behaviors." Curr Opin Neurobiol 23(5): 819-823.
- Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, Panzano VC, Theobald DL, Griffith LC, Garrity PA (2013). "A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila." Nature 500(7464): 580-584.
- Pirez N, Christmann BL, Griffith LC (2013). "Daily rhythms in locomotor circuits in Drosophila involve PDF." J Neurophysiol 110(3): 700-708.
- Shang Y, Donelson NC, Vecsey CG, Guo F, Rosbash M, Griffith LC (2013). "Short neuropeptide f is a sleep-promoting inhibitory modulator." Neuron 80(1): 171-183.
- Berni J, Pulver SR, Griffith LC, Bate M (2012). "Autonomous circuitry for substrate exploration in freely moving Drosophila larvae." Curr Biol 22(20): 1861-1870.
- Donelson N, Kim EZ, Slawson JB, Vecsey CG, Huber R, Griffith LC (2012). "High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the "tracker" program." PLoS ONE 7(5): e37250.
- Griffith LC (2012). "Identifying behavioral circuits in Drosophila melanogaster: moving targets in a flying insect." Curr Opin Neurobiol 22(4): 609-614.
- Trott AR, Donelson NC, Griffith LC, Ejima A (2012). "Song choice is modulated by female movement in Drosophila males." PLoS ONE7(9): e46025.
- Maiya R, Lee S, Berger KH, Kong EC, Slawson JB, Griffith LC, Takamiya K, Huganir RL, Margolis B, Heberlein U. (2012). "DlgS97/SAP97, a neuronal isoform of discs large, regulates ethanol tolerance." PLoS ONE 7(11): e48967.
- Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Shang Y, Haynes P, Pírez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Nat Neurosci. 2011 Jun 19;14(7):889-95.
- Central Regulation of Locomotor Behavior of Drosophila melanogaster Depends on a CASK Isoform Containing CaMK-like and L27 Domains. Slawson JB, Kuklin EA, Ejima A, Mukherjee K, Ostrovsky L, Griffith LC. Genetics. 2011 Jan; 187(1):171-84.
- Intracellular Regions of the Eag Potassium Channel Play a Critical Role in Generation of Voltage-dependent Currents. Li Y, Liu X, Wu Y, Xu Z, Li H, Griffith LC, Zhou Y. J Biol Chem. 2011 Jan 14;286(2):1389-99.
- Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Nature. 2010 Mar 25;464(7288):597-600.
- Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Pulver SR, Griffith LC. Nat Neurosci. 2010 Jan;13(1):53-9.
- Courtship learning in Drosophila melanogaster: diverse plasticity of a reproductive behavior. Griffith LC, Ejima A. Learn Mem. 2009 Nov 19;16(12):743-50. Print 2009.
- CaMKII uses GTP as a phosphate donor for both substrate and autophosphorylation. Bostrom SL, Dore J, Griffith LC. Biochem Biophys Res Commun. 2009 Dec 25;390(4):1154-9.
- Multimodal sensory integration of courtship stimulating cues in Drosophila melanogaster. Griffith LC, Ejima A. Ann N Y Acad Sci. 2009 Jul;1170:394-8.
- Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. J Neurophysiol. 2009 Jun;101(6):3075-88.
- Channelrhodopsin2 mediated stimulation of synaptic potentials at Drosophila neuromuscular junctions. Hornstein NJ, Pulver SR, Griffith LC. J Vis Exp. 2009 Mar 16;(25).
- High-resolution video tracking of locomotion in adult Drosophila melanogaster. Slawson JB, Kim EZ, Griffith LC. J Vis Exp. 2009 Feb 20;(24).
- Alternative splicing of the eag potassium channel gene in Drosophila generates a novel signal transduction scaffolding protein. Sun XX, Bostrom SL, Griffith LC. Mol Cell Neurosci. 2008.
- Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain.Shang Y, Griffith LC, Rosbash M. Proc Natl Acad Sci U S A. 2008;105(50):19587-94.
- PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, et al. Neuron. 2008;60(4):672-82.
- Sleep: hitting the reset button.Griffith LC, Rosbash M. Nat Neurosci. 2008;11(2):123-4.
- CaMKII: new tricks for an old dog. Griffith LC. Cell. 2008;133(3):397-9.
- Neuroscience - Love hangover. Griffith LC. Nature. 2008;451(7174):24-5.
- Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. Ejima A, Griffith LC. PLoS ONE. 2008;3(9):e3246.
- Modulation of GABA(A) receptor desensitization uncouples sleep onset and maintenance in Drosophila. Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Nat Neurosci. 2008;11(3):354-9.
- A structural mechanism for maintaining the 'on-state' of the CaMKII memory switch in the post-synaptic density. Mullasseril P, Dosemeci A, Lisman JE, Griffith LC. J Neurochem. 2007;103(1):357-64.
- The Drosophila ARC homolog regulates behavioral responses to starvation. Mattaliano MD, Montana ES, Parisky KM, Littleton JT, Griffith LC. Mol Cell Neurosci. 2007;36(2):211-21.
- Generalization of Courtship Learning in Drosophila Is Mediated by cis-Vaccenyl Acetate. Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, et al. Curr Biol. 2007;17(7):599-605.
- Cholinergic neurons mediate CaMKII-dependent enhancement of courtship suppression. Mehren JE, Griffith LC. (2006) Learn Mem. 2006 Nov-Dec;13(6):686-9.
- Activity-dependent gating of CaMKII autonomous activity by Drosophila CASK. Hodge JJ, Mullasseril P, Griffith LC. Neuron. 2006 Aug 3;51(3):327-37.
- Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. Park D, Griffith LC (2006) J Neurophysiol. 2006 Jun;95(6):3955-60.
- Role for calcium/calmodulin-dependent protein kinase II in the p75-mediated regulation of sympathetic cholinergic transmission. Slonimsky JD, Mattaliano MD, Moon JI, Griffith LC, Birren SJ. (2006). Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2915-9.
- Sequential learning of pheromonal cues modulates memory consolidation in trainer-specific associative courtship conditioning. Ejima A, Smith BPC, Lucas C, Levine JD and Griffith LC (2005). Curr Biol. 15:194-206.